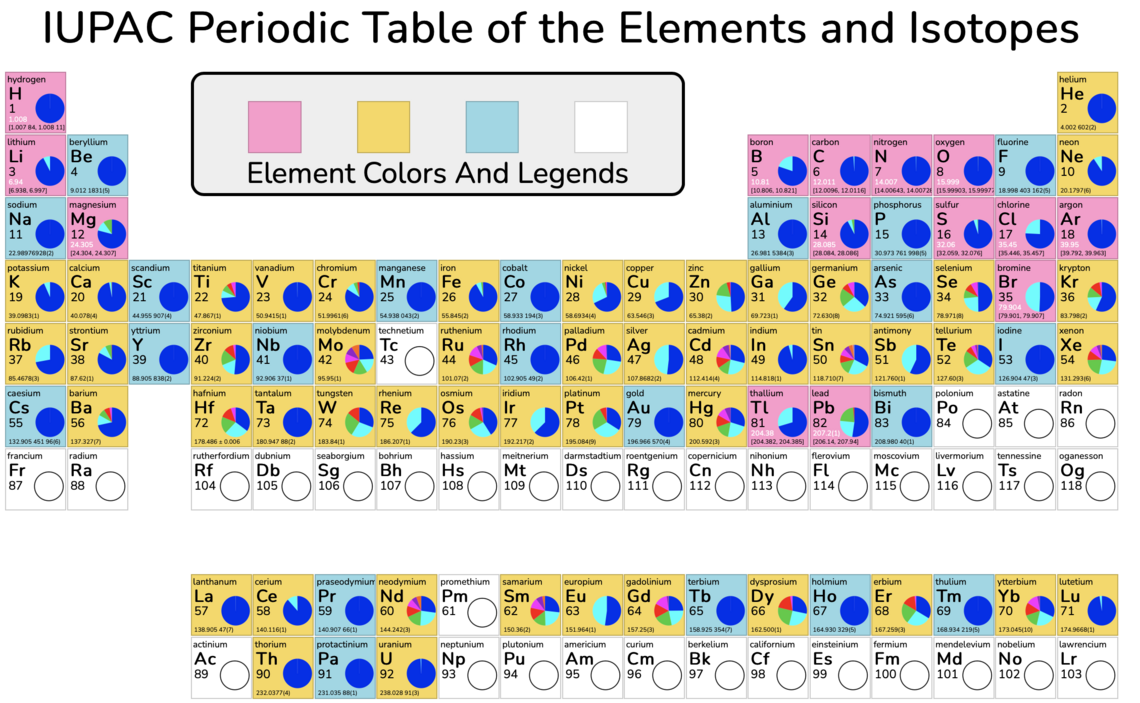
What are isotopes?
Elements such as calcium, iron, copper, and zinc play critical roles in a number of significant biochemical and geochemical processes. For example, changes in the regulation of calcium in a living system may lead to changes in the concentration and isotopic composition of the element metabolized by the organism; the incorporation of copper by metalloproteins is critical in the regulation of this redox sensitive element in the brain; zinc is used extensively in industry and its presence in the environment might be a useful indicator of anthropogenic activities. Further, disruptions in the delicate balance among these elements in living systems may be the cause or also the effect of diseases such as Alzheimer’s, Parkinson’s, and even cancer. A clear understanding of the significance of these elements at the interface between the geosphere and biosphere as well as in vivo can be generated by the development of insightful analytical tools supported by theoretical models that have the ability to distinguish complex processes and pathways.
Each of these elements possesses several stable isotopes and subtle but significant variations in their isotopic compositions are reported in the literature. An isotope of an element has the same number of protons (and hence the same number of electrons in a neutral atom) but a different number of neutrons. Because isotopes have the same electronic configurations, they behave in a similar manner in chemical reactions. However, the differing numbers of neutrons in the nucleus mean that these atoms have different masses and will participate in physical, chemical, and biological reactions at different rates. Therefore, isotopes of an element may be redistributed in a given system and isotopic composition can provide insights into the sources of an element or the actual processes affecting the atoms.
There is an important opportunity to utilize isotopic composition to learn the function of these key elements at the Earth-Human interface with the ultimate goal of applying this knowledge to challenges in health and the environment. The application of reliable isotopic tools is very much nascent for many of these elements. What is needed now is a detailed understanding of the particular biogeochemical processes that actually cause the changes in isotopic composition.

Water in the solid phase, two moles of hydrogen and one mole of oxygen. The ice cubes at the bottom are deuterium with one proton and neutron in each nucleus. The ice cubes at the top are protium with only a single proton in each nucleus.